Nanomaterials that respond to cancer-specific stimuli show potential in the targeted delivery of treatments and imaging compounds, but many challenges remain.
Published : October 26 , 2018 / DOI : 10.1080/14686996.2018.1528850
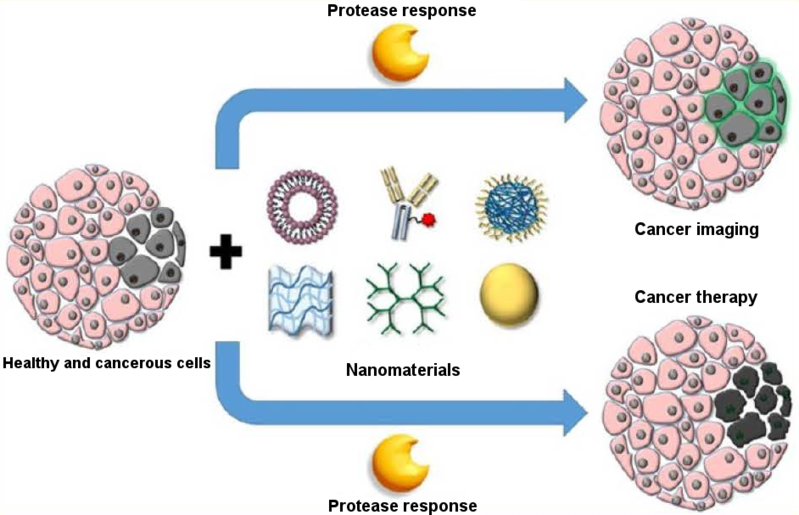
Nanosystems that deliver anticancer drugs or imaging materials to tumours are showing significant progress, particularly those that respond to tumour-related stimuli, according to a review published in the journal Science and Technology of Advanced Materials. However, further research is still required to make sure these delivery systems are stable, non-toxic and biodegradable. Nanocarriers designed to release their contents only in cancerous tissue are of great interest because they can reduce the negative effects of chemotherapeutic agents on healthy tissues. They can also deliver contrast materials to the tumour for enhanced imaging. Nanomaterials are being designed to specifically target tumours by responding to unique tumour conditions, such as acidity and over-expressed enzymes, explain the reviewers from Xiamen University in China. Acidity levels normally vary between tissue types, and tumours are usually more acidic than the surrounding healthy tissue. Researchers are using this to design delivery vehicles from organic, inorganic and hybrid nanomaterials that release their contents in response to tumours’ acidic environment. For example, acid-sensitive polymers have been explored for delivering the chemotherapeutic drug doxorubicin coupled with a fluorescent compound. When the system reaches its target, it is taken up by tumour cells and is exposed to their acidic environment. The polymer then changes its shape in a way that releases its contents, allowing both the treatment and imaging of the cancerous cells. Reduction potential is also being used as a stimulus for cancer-targeting delivery vehicles. Reduction potential is the measure of the tendency of molecules to acquire electrons and thus become ‘reduced’. Reduction potential is different in cancerous tissue than it is in healthy tissue. Nanocarriers are being designed that break down when exposed to a reducing agent in the body called reduced state glutathione tripeptide, which is 1,000 times higher inside tumour cells than outside. This type of nanocarrier has excellent stability in the blood and rapidly responds to the reducing environment in tumours. Some have already been approved by the U.S. Food and Drug Administration for use in clinics. Other nanocarriers respond to enzymes that are abnormally expressed inside tumours. They are made of materials that these enzymes can break down, thus releasing the contents. The breakdown of the nanocarrier wall can be so effective in some cases, it might reduce the amount of drug needed to have a therapeutic effect. Despite the promise these nanomaterials show, more work is needed. Acid-responsive carriers, for example, need to have a better drug-loading capacity, stability, and biodegradability. Further examination of the toxicity of enzyme-responsive materials is also required. “Considering the promising potential of stimuli-responsive nanomaterial, much more efforts should be made to fabricate more platforms for triggered drug delivery with increased efficiency and reduced side effects for cancer therapy,” the researchers conclude. About Science and Technology of Advanced Materials Open access journal, STAM publishes outstanding research articles across all Aspects of materials science, including functional and structural materials, theoretical analyses, and properties of materials. For more information, please contact: Mikiko Tanifuji STAM Publishing Director Tanifuji.Mikiko@nims.go.jp
Information
- Authors
- Lei Li, Junqing Wang, Hangru Kong, Yun Zeng & Gang Liu
- Citation
- Sci. Technol. Adv. Mater.19(2018)771.
- URL
- http://doi.org/10.1080/14686996.2018.1528850